A New Hope Against an Old Killer
Tuberculosis remains one of the most deadly infectious diseases in the world. Despite decades of research, millions are still infected each year and more than a million people die annually. A new antibiotic called sorfequiline is now raising hopes that treatment could become faster, more effective and easier for patients to complete.
Recent trial results presented at the Union Conference on Lung Health in Copenhagen suggest that sorfequiline offers stronger action against the tuberculosis bacteria than existing standard therapies, while maintaining a comparable safety profile. If further trials confirm these findings, sorfequiline could help transform the way TB is treated in both drug sensitive and possibly drug resistant cases.
The Global Burden of Tuberculosis
According to recent estimates, about 10.7 million people developed tuberculosis last year and approximately 1.23 million died from the disease. In its annual report, the World Health Organization again classified TB as a major global public health problem and the leading infectious cause of death.
Efforts to control TB are under pressure. Progress towards the United Nations goal of ending tuberculosis as a public health threat this decade is already behind schedule. Funding gaps, health system strain, and cuts in aid threaten to reverse hard won gains. Against this backdrop, any new treatment option that can increase cure rates and shorten therapy is especially significant.
Short Summary Table
Key Detail |
Information |
|---|---|
Disease Focus |
Tuberculosis (TB), a leading infectious cause of death worldwide |
New Drug Name |
Sorfequiline |
Main Benefit Shown |
Stronger action than existing TB treatments with similar safety profile |
Trial Scale |
309 participants across 22 sites in 5 countries |
Patient Type in Trial |
Drug sensitive tuberculosis patients |
Potential Use |
Universal regimen for TB patients, including possible drug resistant cases |
Current Standard Treatment |
Around 6 months, about 90 percent success for drug resistant TB |
Key Advantage |
Shorter, simpler treatment with fewer side effects and fewer clinic visits |
Development Stage |
Successful trial presented, phase 3 trial planned for 2026 |
Official TB Information Website |
Sorfequiline: A New Antibiotic With Stronger Action
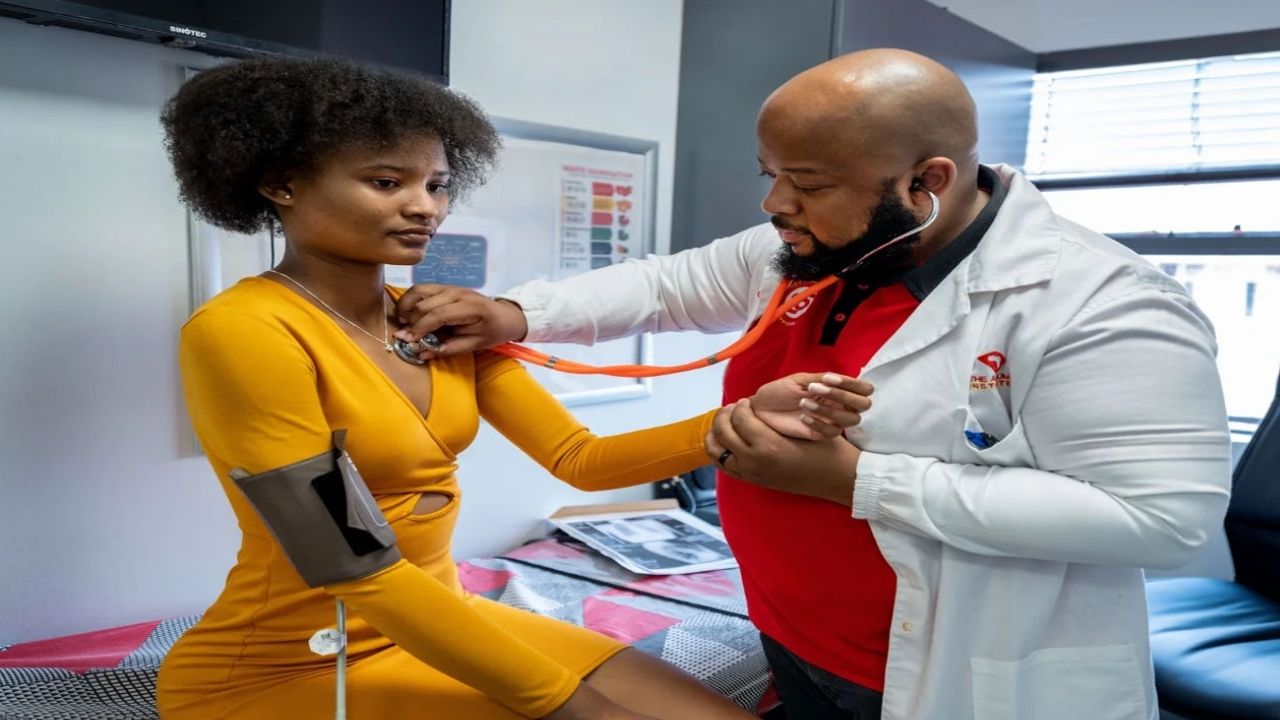
Sorfequiline is a new antibiotic being developed to target the bacteria that cause TB. Researchers from the TB Alliance, a non profit organization focused on TB drug development, presented early trial data indicating that sorfequiline has stronger antibacterial activity than existing standard regimens used for drug sensitive TB.
The crucial point is that this stronger action did not come at the cost of safety. The safety profile in the trial was broadly comparable to that of current therapies, which is essential if the drug is to be used widely. A powerful but intolerable drug would not be helpful in real world settings, where patients already struggle with long and sometimes difficult treatment regimens.
Inside The Trial: Design And Locations
The sorfequiline trial included 309 participants and was conducted across 22 clinical research sites in five countries: South Africa, the Philippines, Georgia, Tanzania and Uganda. All participants had drug sensitive tuberculosis, meaning their infection could usually be treated with the standard cocktail of existing medicines.
Different dose regimens of sorfequiline based combinations were tested to understand the best balance of efficacy, safety and practicality. The trial explored how sorfequiline could be integrated into a treatment plan that aims to either match or exceed current cure rates while reducing the duration and complexity of therapy.
Towards A Universal, Faster TB Regimen
One of the most exciting ideas to emerge from the study is the possibility of using a sorfequiline based regimen as a universal treatment for TB. Dr Maria Beumont, vice president of TB Alliance, explained that the goal is to have a regimen that can be started in any patient who tests positive for TB, even before detailed laboratory results are available.
Today, doctors often have to wait days or even weeks for a full assessment of whether the infection is drug sensitive or drug resistant. In many parts of the world, access to rapid diagnostic tests is limited or inconsistent. A universal regimen could allow clinicians to start effective treatment immediately, without waiting for detailed classification of the TB type.
This would simplify decision making and could help ensure that patients begin appropriate therapy sooner, which is crucial for both individual recovery and public health.
Impact On Health Systems And Patient Experience
Shorter, more effective and less toxic treatments can bring benefits well beyond the individual patient. Dr William Brumskine, a clinical research site leader at the Aurum Institute in Rustenburg, South Africa, highlighted that a universal, shorter regimen with fewer side effects could reduce the number of clinic visits required.
If patients need fewer follow ups and experience fewer adverse reactions, health care providers can focus more time and attention on those who need extra support. This could improve overall quality of care while reducing system burdens. Fewer clinic visits also mean less time away from work or family, and lower transport costs for patients, which can improve adherence to treatment.
From Gruelling Regimens To Shorter Courses
To understand why sorfequiline is generating enthusiasm, it is useful to look at how TB treatment has evolved. A decade ago, drug resistant TB often required 18 months or more of therapy, including painful injections, lengthy hospital stays and a long list of side effects. Even with such effort, cure rates were only about 50 percent.
In 2019, a new gold standard regimen was introduced for many forms of drug resistant TB. This newer approach can cure around 90 percent of patients within about six months, which represents major progress. Researchers now hope that sorfequiline based regimens can push outcomes even further, potentially increasing cure rates, shortening treatment time and reducing toxicity.
Early Signals And Next Steps In Development
Researchers involved in the trial have reported anecdotal stories of patients who appeared to respond remarkably quickly to treatment. Some site teams described cases where individuals were cured much faster than expected, even though they did not know which treatment arm the patient had been assigned to at the time. These anecdotes helped build anticipation ahead of the formal presentation of results.
The TB Alliance has indicated that it hopes to begin a large phase 3 clinical trial of sorfequiline in 2026. Phase 3 trials are critical because they involve larger patient populations and are designed to confirm effectiveness and safety in more diverse real world conditions. Only after successful phase 3 trials and regulatory review could sorfequiline become widely available as part of standard TB regimens.
Caution And Concerns About Universal Use
While the potential advantages are clear, experts also warn about possible downsides of using a powerful universal regimen for all TB cases. Dr Kavindhran Velen, chief scientific officer at the International Union against Tuberculosis and Lung Disease, noted that a shorter and more effective regimen could significantly increase the proportion of patients who complete therapy and reduce the time they remain infectious.
At the same time, he raised concerns that relying too heavily on a single universal regimen might reduce incentives for health systems to invest in broader innovations, such as improving laboratories, diagnostic testing and surveillance. He also cautioned against using a very strong drug on patients who might be cured with a gentler regimen, comparing it to taking a hammer to an ant.
The key will be to balance the power of a universal regimen with careful stewardship, so that patients are not overexposed to heavy treatment when it is not necessary, and drug resistance is not encouraged.
Frequently Asked Questions
1. What is sorfequiline in tuberculosis treatment?
Sorfequiline is a new antibiotic being developed to treat tuberculosis. Early trial data suggest it has stronger action against TB bacteria compared with existing standard treatments for drug sensitive TB, while showing a comparable safety profile.
2. How many people took part in the sorfequiline trial?
The trial included 309 participants across 22 clinical sites in South Africa, the Philippines, Georgia, Tanzania and Uganda. All participants had drug sensitive TB.
3. Can sorfequiline be used for drug resistant TB?
The trial focused on drug sensitive TB, but researchers believe that TB infections that are resistant to standard treatment could also benefit from a sorfequiline based regimen. Further studies will be needed to confirm its role in drug resistant cases.
4. How could sorfequiline change the duration of TB treatment?
A decade ago, some drug resistant TB regimens lasted 18 months or more. Current gold standard treatment now cures many patients in about six months. Researchers hope that sorfequiline will allow for even shorter and more effective regimens, although exact treatment durations will depend on results from future phase 3 trials.
5. When could sorfequiline become widely available?
The TB Alliance aims to launch a phase 3 clinical trial in 2026. If that trial and subsequent regulatory reviews are successful, sorfequiline could eventually become part of standard TB treatment in the years that follow. The exact timeline will depend on trial outcomes and approval processes in different countries.
Conclusion
The emergence of sorfequiline as a promising new antibiotic for tuberculosis represents an important step forward in the fight against one of the world’s deadliest infectious diseases. Early trial results suggest that sorfequiline can deliver stronger action than existing treatments while preserving a similar safety profile.
If future trials confirm its benefits, sorfequiline could help create a universal, shorter and more tolerable TB regimen that improves cure rates, eases pressure on health systems and reduces the burden on patients. At the same time, experts urge caution to ensure that this powerful tool is used wisely, alongside continued investment in diagnostics and broader TB control strategies.
For now, the global health community will be watching closely as the TB Alliance prepares for phase 3 trials and as researchers explore how best to integrate sorfequiline into the evolving landscape of TB treatment.
For More Information Click HERE